- CVD diamond is the chemical vapor deposition diamond (also named CVD diamond films, diamond thick films). It is another significant revolution in synthetic diamond fields after the synthesis of diamond under high temperature and high pressure. Its size can be great, which is reaching 120mm diameter and 2mm thickness in mass production at present. And the crystal quality can be compared with natural IIa diamond. At a low-pressure condition, the gases containing carbon such as CH4 and C3H8 will pyrolysis. Then with the effect of activation radical as H+ and OH, pure polycrystal diamond will be deposited on solid substrates. The main synthesis methods of CVD diamond films include: Hot-filament CVD, DC Arc Plasma Jet CVD, Microwave plasma CVD, DC hot cathode plasma CVD and the like. Among those, DC Arc Plasma Jet CVD has the performance of fast speed and good quality, which is very suitable for the production of tool-level diamond thick films.
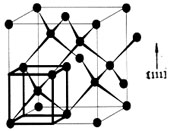
- CVD diamond is the face-centered cubic structure, as shown in the following Fig. The diamond Bravais cell includes two carbon atoms. In a face-centered cubic cell, there are four carbon atoms located at the 1/4 position of space diagonal respectively. Every carbon atom is covalent with surrounding 4 carbon atoms. One is at the center of the regular tetrahedron. and the valence bond orientations of the four carbon atoms at the center of the regular tetrahedron are different from those of carbon atoms at apex angle. It makes the surrounding circumstance of carbon atoms at the cubic apex angle and face-center are vary from the four carbon atoms at the diagonal. Therefore, though diamond is composed by carbon atoms(nature diamond contain 98.9% 12C and 1.1% 13C) only, its structure is a duplex type grid formed by the overlapping of two face-centered cubic son cells which shift along each space diagonal 1/4 length respectively. The crystal lattice constant is 3.56A and the bond length is 1.54A. Because of the compact crystal structure, small carbon atom radius and short bond length, diamond is extremely hard.
- Diamond can be divided into Ⅰa、Ⅰb、Ⅱa、Ⅱb in terms of physical property. In theⅠa diamond, the nitrogen concentration is high(about 1/1000 order of magnitude) and exists as anti-paramagnetic congeries. Such diamond has good mechanical properties, high electrical insulation and narrow light transmission scope. The nitrogen concentration in Ⅰb diamond is a little high and exists as paramagnetic mode. The thermal conductivity of Ⅰb diamond is better than that of Ⅰa diamond. Ⅱa diamond has an extremely low nitrogen concentration, the highest thermal conductivity and the widest transmission wave band. Ⅱb diamond contains boron and has the character of P type semiconductor. At present, Ⅱb diamond can be synthesized by CVD.
|
 |


